What is a bioreactor?
A bioreactor is a vessel or device designed to provide a controlled environment for the growth of living cells or microorganisms. In simple terms, it’s like a specialized tank where biological processes (such as cell culture or fermentation) are carried out under optimal conditions to produce a desired product. Bioreactors maintain factors like temperature, pH, nutrient supply, and oxygen at ideal levels so that cells can “focus” on doing their job, growing and producing target compounds (be it a medicine, enzyme, or food product). They range in size from small bench-top jars to large industrial tanks holding thousands of liters, often with a stainless steel bioreactor design for durability at scale. (When used for microbial fermentation, a bioreactor is often called a fermentor, especially in brewing or antibiotic production).
Bioreactors for cell culture are widely used in biotechnology and medicine, for example, to cultivate animal cells that produce vaccines, antibodies, or therapeutic proteins. In other cases, bioreactors (fermenters) are employed to grow microbial cultures for processes like brewing beer, fermenting dairy products, or producing biofuels. In this basic guide, we’ll answer common questions about bioreactors, including how they work, why they’re used, their key components and types (from stirred tank reactors to newer single-use systems), and how scaling up bioreactor processes is achieved.
Why should you consider using a bioreactor?
Culturing cells in a bioreactor offers several benefits over traditional methods (like shake flasks or Petri dishes in an incubator). Bioreactors are designed to create optimal growth conditions and solve the limitations of simpler culture techniques. Some key advantages of using bioreactors include:
Higher cell yield (scalability): Bioreactors are scalable, meaning you can increase the culture volume in one vessel instead of running dozens of small flasks. A stirred-tank bioreactor can handle working volumes of thousands of liters, whereas regular shake flasks are at most a few liters. This makes scaling up a bioreactor process much more efficient for producing large quantities of cells or products in one go.
Better control & consistency: Bioreactors tightly monitor and regulate critical parameters (like pH, temperature, dissolved oxygen, and nutrient levels) in real time using built-in sensors and automated controls. In a simple flask, usually only temperature (in an incubator) is controlled, but a bioreactor can automatically adjust conditions (e.g. pump in oxygen or nutrients) to keep cells happy. This leads to more consistent growth and less batch-to-batch variability in product quality. Every batch has a better chance to succeed because the environment remains optimal and reproducible.
Faster optimization and research: Modern bioreactor systems allow multiple small cultures to run in parallel, which is great for testing different conditions quickly. Scientists can experiment with different parameters (like varying pH or feed rate) simultaneously in miniature bioreactors to find the best recipe for cell growth or production. This high-throughput approach saves time in process development compared to doing one flask at a time.
In summary, bioreactors enable large-scale cultivation with tight environmental control, improving yields and consistency. They bridge the gap from lab bench to industrial production by creating conditions that are difficult to achieve in basic shake flasks or petri dishes. Bioreactors also provide a closed, sterile system, reducing contamination risk and allowing cells to be grown for longer durations or to higher densities than in open cultures.
What are the main components of a bioreactor?
Even though bioreactors come in many designs, most share a set of core components that allow them to maintain a suitable growth environment. Focusing on the common stirred-tank bioreactor (the typical workhorse in labs and industry), the key parts include:
Vessel (tank): The main container that holds the culture broth (cells plus nutrient medium). It is typically a cylindrical tank designed to be airtight and sterile. Vessels can be made of stainless steel, glass, or plastic, depending on scale and usage. Industrial bioreactors usually use stainless steel vessels for durability, ease of cleaning, and sterility. Lab-scale units might use glass or single-use plastic liners. The vessel often has a heating/cooling jacket or coils to regulate temperature.
Agitation system (impeller): Most bioreactors have a mechanical agitator – commonly an impeller (a spinning blade or paddle) mounted on a shaft, driven by a motor from the top or bottom of the vessel. The impeller stirs the liquid culture, mixing nutrients, gases, and cells uniformly. Proper agitation ensures all cells receive equal access to food and oxygen, and it prevents temperature gradients within the tank. Baffles (stationary plates on the vessel wall) may be present to improve mixing. The mixing speed (RPM) can be adjusted depending on the organism’s needs: for example, microbial cultures often tolerate faster stirring than fragile mammalian cells.
Aeration system (sparger): Cells that require oxygen (which is most cultures except strict anaerobes) need a steady gas supply. A sparger is an air (or oxygen) inlet device, usually a ring or tube with fine holes, at the bottom of the vessel. It bubbles air or oxygen into the culture in the form of small bubbles. These bubbles provide oxygen to the cells and help carry away carbon dioxide. The combination of sparging (bubbling) and stirring helps maximize gas exchange in the liquid. In some systems (like airlift bioreactors), aeration also contributes to mixing the culture.
Temperature control: Bioreactors must keep the culture at the right temperature for the cells (for example, 37 °C for human cells, or around 30 °C for yeast). Temperature is regulated via built-in systems such as heating/cooling jackets around the vessel or internal coils through which temperature-controlled water circulates. Sensors continuously measure the temperature, and the control system turns heaters or chillers on/off to maintain the setpoint. Keeping temperature stable is crucial, since even small fluctuations can affect cell growth and product formation.
Sensors and control system: A modern bioreactor is equipped with various sensors to monitor conditions inside the vessel. For example, probes measure pH (acidity), dissolved oxygen (O₂ level in the liquid), and sometimes cell density or glucose concentration. There are also typically foam sensors and level sensors. These measurements feed into a control system (a computer or controller unit) that automatically adjusts parameters to keep conditions optimal.
For instance, if pH drops as cells produce acidic byproducts, a pump can add base to neutralize it; or if oxygen is low, the controller can increase the airflow or agitation speed. This feedback loop allows the bioreactor to maintain a stable environment (homeostasis) for the culture. Operators can set the desired values (setpoints) and the bioreactor manages itself, with data often logged for analysis.
Sterility and ports: Bioreactors are designed for aseptic operation, meaning they must prevent contamination by unwanted microbes. Vessels have sealed ports or connectors for adding liquids (like nutrients or acid/base for pH control), sampling the culture, or harvesting product, all without letting contaminants in. Large stainless steel bioreactors are usually equipped with sterilization systems (they can be steam-sterilized in place between batches).
In contrast, single-use bioreactors come pre-sterilized (via gamma irradiation) and are simply thrown away after use, eliminating the need for cleaning/sterilizing between runs. Many units also include foam control mechanisms (a sensor and an automated antifoam chemical addition) because aeration and stirring can cause foam that needs to be managed so it doesn’t overflow or foul gas outlets.
What types of bioreactors are there?
Bioreactors come in various types and designs to suit different organisms and processes. The stirred-tank bioreactor (STR) we described above is the most common design – a cylindrical tank with mechanical mixing. But other bioreactor configurations are used in special situations. Here are some common types of bioreactors and their characteristics:
Stirred-Tank Bioreactors (STRs): This is the standard bioreactor design and is widely used across industries. It consists of a tank (often cylindrical) with an impeller for mixing the culture. STRs provide excellent control over environmental parameters (temperature, pH, oxygen, etc.) and are very versatile. They are used for processes ranging from microbial fermentations (e.g. producing antibiotics and enzymes) to mammalian cell cultures for biopharmaceuticals. In chemical engineering terms, an STR is similar to a stirred-tank reactor model, and it can be operated in batch, fed-batch, or continuous modes.
Airlift Bioreactors: In an airlift bioreactor, there is no mechanical stirring shaft; instead, mixing is achieved by pumping air (or gas) into the base of the reactor. The rising gas bubbles create circulating liquid flow that stirs the culture. This design has lower shear stress on cells compared to an impeller, which is beneficial for fragile cells (e.g. many mammalian cell cultures can be sensitive to excessive stirring). Airlift bioreactors often have an internal draft tube to direct flow. They are commonly used for cultivating cells for vaccines or tissue engineering, where gentle handling of cells is required.
Photobioreactors: These bioreactors are specialized for photosynthetic organisms like algae or cyanobacteria. A photobioreactor provides a light source in addition to the usual bioreactor controls. Designs can be tubular (arrays of clear tubes), flat panels, or columnar, to maximize the surface area exposed to light. Algae grown in photobioreactors can capture CO₂ and convert it to biomass, and are used to produce biofuels, nutritional supplements, and other valuable compounds (like pigments or omega-3 fatty acids). Photobioreactors must balance light delivery with mixing and gas exchange, and often require temperature control due to heating from the lights.
Packed-Bed (Fixed-Bed) Bioreactors: In a packed-bed bioreactor, cells or enzymes are immobilized on a solid support material (such as beads, fibers, or porous resin) packed into a column or vessel. The nutrient medium flows through the packed bed to supply the immobilized cells with nutrients and remove products. This setup allows extremely high cell densities and long reaction times, since cells are retained in the reactor.
Packed-bed bioreactors are used, for example, for enzymatic reactions, certain fermentation processes, or growing plant cells that attach to support matrices. They are often operated continuously (fresh medium in, product out). A related design is the fluidized-bed bioreactor, where the particles are suspended and mixed by the flow of liquid or gas.
| Bioreactor Type | Mixing / Operation | Key Advantages | Typical Applications |
|---|---|---|---|
| Stirred-Tank Bioreactor | Mechanical impeller stirs the culture | Excellent control of temperature, pH, oxygen; versatile operation modes (batch, fed-batch, continuous) | Microbial fermentations (antibiotics, enzymes); mammalian cell culture for biopharma |
| Airlift Bioreactor | Gas sparging drives circulation (no shaft) | Low shear stress; gentle on fragile cells | Vaccine production; tissue engineering; delicate mammalian cell cultures |
| Photobioreactor | Light delivery + mixing and gas exchange | Enables photosynthesis; controlled light & temperature | Algae cultivation; production of pigments, omega-3s, and biofuels |
| Packed-Bed Bioreactor | Medium flows through immobilized support | Very high cell density; long residence times | Enzymatic reactions; certain fermentations; plant cell suspensions |
What is the difference between single-use and stainless steel bioreactors?
In modern bioprocessing, there are two major categories of bioreactor hardware: traditional stainless steel bioreactors (made of rigid metal, and designed to be cleaned and reused for years) and single-use bioreactors (made of disposable plastic bags or liners, intended for one-time or single-campaign use). Both types can function similarly in cultivating cells, but they have important differences:
Materials and reusability: Stainless steel bioreactors are durable, permanent vessels that must be cleaned and sterilized between batches (often through automated Clean-In-Place/Steam-In-Place cycles). Single-use systems, by contrast, use pre-sterilized plastic bags or containers as the culture vessel, which are thrown away after use. This means single-use units don’t require lengthy cleaning or sterilization procedures – a fresh sterile bag is simply installed for each run.
Scale and capacity: Stainless steel reactors dominate at very large scales – they can be built in sizes of 10,000–20,000 L or more for huge production batches. Single-use bioreactors are typically available up to about 2,000 L working volume (with some newer designs reaching ~5,000 L). Very high cell density processes can somewhat compensate for the volume limit, but generally stainless steel is preferred for the largest-volume manufacturing (such as blockbuster drug production).
Turnaround time and flexibility: Single-use bioreactors have a quicker turnaround between batches since you eliminate the cleaning/sterilizing step – you can simply swap in a new sterile liner and start the next run. This fast turnover is great for multi-product facilities or rapid clinical production, and it reduces downtime. Stainless steel systems have slower turnaround due to cleaning, and they require utilities (steam, water) to support those cleaning processes. However, stainless steel may handle certain processes (e.g. some microbial fermentations with harsh conditions) better, and you don’t have to worry about supply of disposable bags.
Contamination and validation: Single-use technology can reduce cross-contamination risk because each batch uses a brand-new sterile contact surface. There is no risk of residue carryover from a previous batch if the bag is discarded. In contrast, stainless steel requires rigorous cleaning validation to ensure no contaminants or product residues remain. On the other hand, single-use plastics must be tested for leachables and extractables (chemicals that might leach out of the plastic into the culture) as part of validation, whereas steel vessels don’t have that issue.
Costs and waste: A stainless steel bioreactor involves a high up-front capital cost to install (plus the ongoing costs of cleaning chemicals, water, energy, and maintenance). Single-use bioreactors generally have lower initial equipment costs and can be deployed faster (no need for complex pipework or CIP systems), but they incur higher recurring costs for disposable bags, filters, and plastic components each run.
From an environmental perspective, single-use systems generate a lot of plastic waste, while stainless steel systems generate wastewater and chemical waste from cleaning processes. Sustainability comparisons are complex; single-use saves water and energy that would be used for cleaning, but creates plastic biohazard waste that must be disposed of.
In practice, the choice between single-use and stainless steel depends on the situation: Many new biotech facilities favor single-use for flexibility (especially for processes ≤2000 L or multi-product pipelines), whereas large-scale production of a single product (e.g. a high-demand vaccine) might still use giant stainless steel tanks. Some facilities even use a hybrid approach, using disposable bioreactors for certain steps and stainless steel for others, to get the benefits of both. The good news is that from the user’s perspective, operating a single-use bioreactor vs a stainless steel bioreactor is very similar – the cells don’t know the difference, as long as the control systems maintain the right environment!
Which cell types can be cultured in bioreactors?
Bioreactors are versatile tools that can grow many different types of cells. Any cell that can benefit from a controlled environment might be a candidate. In general, bioreactors are used for culturing: microorganisms (like bacteria, yeast, and fungi), animal cells (such as mammalian or insect cells), as well as certain plant cells and even algae. Here are a few major categories of cell types grown in bioreactors:
Microbial cells (bacteria and yeast): A huge portion of industrial bioprocesses involve microbial cultures. Bacteria (e.g. E. coli) and yeasts (e.g. Saccharomyces cerevisiae) grow rapidly and are often fermented in bioreactors to produce products like insulin, enzymes, amino acids, ethanol, antibiotics, and more. Many foods and beverages are made via microbial bioreactors – for instance, yeast in bioreactors ferments sugar to brew beer and wine, and bacteria cultures are used to produce yogurt and cheese. Microbial cells typically can tolerate vigorous conditions (higher agitation and aeration rates), and fermenters for microbes were among the first large-scale bioreactors developed.
Animal cells (mammalian and insect cell cultures): Bioreactors are critically important for growing more complex mammalian cells – such as Chinese Hamster Ovary (CHO) cells, HEK 293 cells, or even human immune cells – especially in the biopharmaceutical industry. These cells can produce complex therapeutic proteins like monoclonal antibodies, hormones (e.g. erythropoietin), or vaccines that bacteria cannot make properly. Because mammalian cells are delicate and require very specific conditions (37 °C, precise oxygen and pH control, growth factors, etc.), bioreactors provide the needed stable environment.
Insect cells (like Sf9 or Sf21 cell lines used with the baculovirus expression system) are also cultured in bioreactors to produce vaccines (e.g. some flu vaccines) or gene therapy vectors. Bioreactors allow these animal cell cultures to be grown in suspension in large volumes with controlled mixing and aeration, often using gentler impellers or specialized spargers to accommodate their sensitivity.
Plant cells and algae: It may surprise some, but plant cell suspensions can be grown in bioreactors as well. Plant cell cultures are used to produce secondary metabolites (like pharmaceutical compounds, flavors, or dyes) that whole plants make in small quantities. For example, precursors to anti-cancer drugs or vanilla flavor can be produced from plant cells in a bioreactor. Algae (which are single-celled plants) are cultured in photobioreactors to produce biomass for biofuels or high-value compounds (such as omega-3 fatty acids and pigments).
These photosynthetic cultures need light in addition to the usual nutrient and environmental controls. Bioreactor cultivation of plant cells and algae provides a way to harness their biochemical capabilities on an industrial scale, under sterile and controllable conditions (as opposed to, say, farming algae in open ponds where contamination and weather can be issues).
In short, bioreactors for cell culture can handle everything from tiny microbes to more complex eukaryotic cells. Researchers even use bioreactors for growing tissue constructs or stem cells in regenerative medicine. The choice of bioreactor setup (stirred tank, wave-mixed, etc.) may differ depending on cell type (for example, mammalian cells often use gentler agitation and specialized media). But across the board, if you need to grow a large amount of any cell safely and efficiently, a bioreactor is the tool to use.
| Cell Types | Examples | Culture Conditions | Typical Products | Bioreactor Formats |
|---|---|---|---|---|
| Microbial cells | E. coli, S. cerevisiae, fungi | 20–37 °C; high agitation & aeration | Insulin, enzymes, amino acids, ethanol, yogurt, beer | Stirred-tank fermenter |
| Mammalian cells | CHO, HEK 293, human immune cells | 37 °C; low shear; precise pH, DO control | Monoclonal antibodies, vaccines, hormones | Single-use or stainless-steel STR |
| Insect cells | Sf9, Sf21 (baculovirus system) | ~27 °C; gentle mixing; growth factors | VLPs, recombinant proteins, growth factors | Airlift or gentle-impeller STR |
| Plant cells | Nicotiana sp., Cinchona sp. | 25–30 °C; moderate mixing; dark/light cycles | Secondary metabolites, vaccines | STR or packed-bed |
| Algae | Chlorella, Spirulina | Light + CO₂ supply; moderate mixing | Pigments, biofuels, dietary supplements | Photobioreactor |
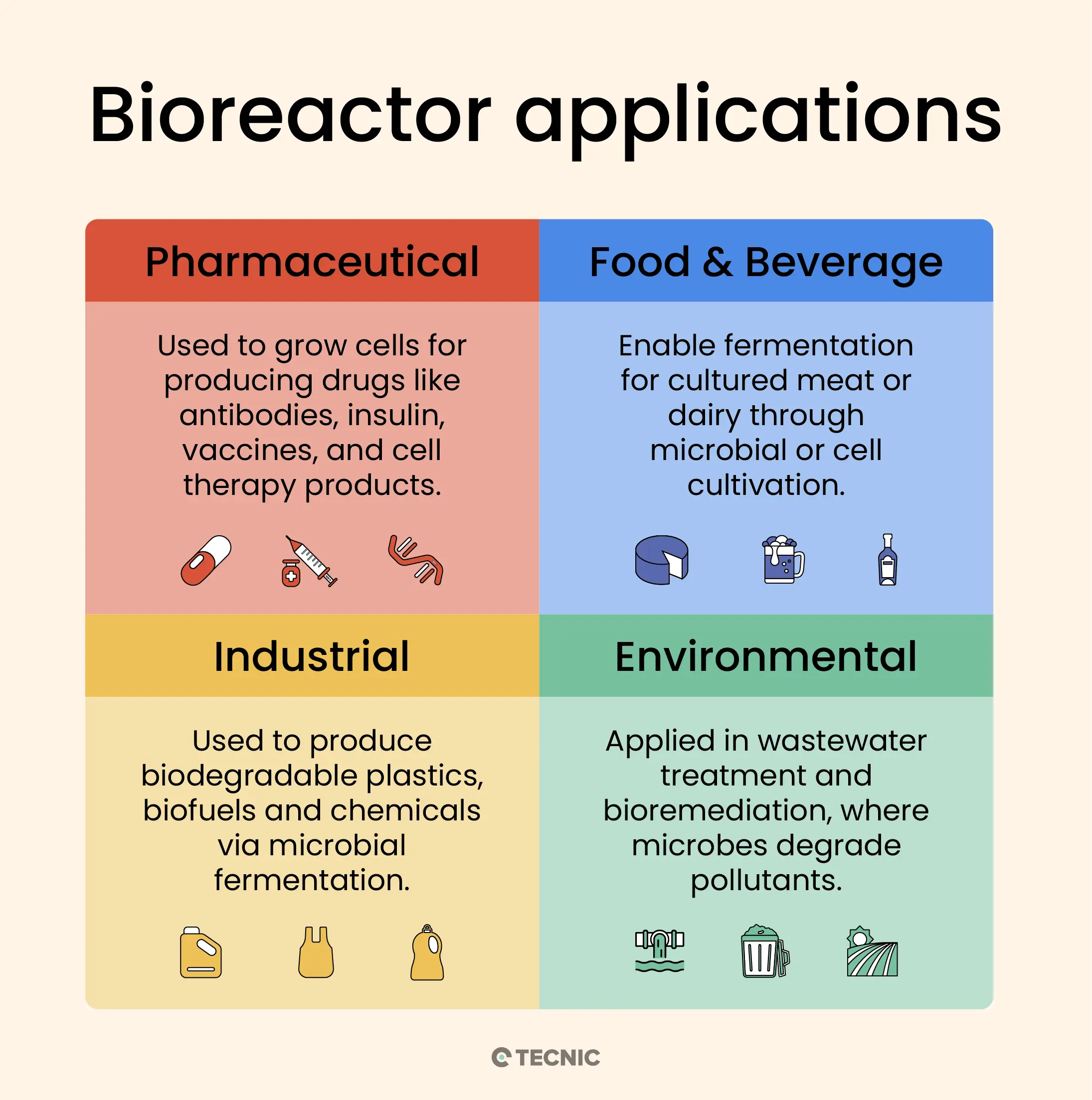
What are typical application areas for bioreactors?
Bioreactors have become indispensable across various industries. Here are some of the main application areas and examples of what bioreactors are used for in each:
Pharmaceutical and Biotechnology (Medical): Perhaps the most headline-making use of bioreactors is in the production of biopharmaceuticals – medical products made by living cells. This includes the manufacture of monoclonal antibodies, therapeutic proteins (like insulin, growth factors), vaccines (including modern cell-culture-based vaccines), gene therapy vectors, and cell therapy products.
Pharma companies grow mammalian or insect cells in large bioreactors to produce these complex biological medicines in high volume. Bioreactors are also used in research and development to scale up promising cell-based processes from the lab to pilot scale. In the emerging field of regenerative medicine, bioreactors can help grow tissues or cultured cells for therapy (for example, culturing CAR-T cells for cancer treatment).
Food and Beverage: Long before the term “bioreactor” was coined, people were using large vats to ferment foods and drinks – which essentially are bioreactors. Today, the food and beverage industry uses bioreactors (often called fermenters) for processes like brewing beer, making wine, fermenting dairy products (yogurt, kefir), producing vinegar, and baking (yeast fermenting dough).
For example, modern breweries use stainless steel fermentation tanks with precise temperature control – these are bioreactors optimized for yeast. Bioreactors are also used to produce food additives like amino acids, vitamins, flavor compounds, and enzymes via microbial fermentation. An exciting new area is cultured meat, where animal muscle cells are grown in bioreactors to produce meat without raising livestock – this technology is still emerging, but early prototypes of cultured meat (and cultured dairy proteins) rely on bioreactor cultivation of animal cells.
Industrial Biotechnology (Chemicals & Biofuels): Bioreactors are employed to manufacture a variety of bio-based chemicals. For instance, certain microbes are cultured to produce biofuels like ethanol and biodiesel (from algae or yeast) in large fermentation tanks. They’re also used for making biopolymers (biodegradable plastics like PLA or PHB produced by bacterial fermentation) and platform chemicals (basic chemical building blocks like citric acid, lactic acid, or amino acids) in bulk.
These processes take advantage of microbes that can convert sugars or other feedstocks into valuable chemicals inside bioreactors. Compared to traditional chemical synthesis, bioprocesses can sometimes be more sustainable and use renewable resources. Bioreactors are key to scaling up these microbial processes to an industrial scale.
Environmental Applications: Bioreactors also play roles in environmental management. In wastewater treatment, for example, microbes in bioreactors break down sewage or industrial waste – specialized bioreactors like activated sludge tanks, biofilters, or anaerobic digesters treat water by biodegrading pollutants. A trickling filter bioreactor is a common setup where wastewater trickles over a bed of rocks or plastic media coated with biofilm, and microbes digest the waste as it passes. Anaerobic bioreactors (sealed tanks without oxygen) are used to treat waste and also to produce biogas (methane) from organic waste.
Another environmental use is in bioremediation, where bioreactors cultivate bacteria or fungi that can consume toxic compounds (like in cleaning up oil spills or chemical waste in a controlled reactor). These applications highlight that bioreactors aren’t just for making products – they’re also used to destroy or transform unwanted substances in an eco-friendly way.
As these examples show, bioreactors are at the heart of modern bioprocessing across diverse sectors – from making life-saving drugs and sustainable fuels to brewing our beer and treating our waste. Whenever we harness biology on a large scale, there’s usually a bioreactor involved.
Which scales of production can bioreactors be used for?
Bioreactors are utilized at multiple scales of production, from tiny lab experiments all the way up to factory-sized manufacturing. They serve as a scalable technology: a process that works in a small bioreactor can often be scaled up to a larger volume (with careful engineering). We typically distinguish bioreactor scales as follows:
Small scale (laboratory scale): Micro-bioreactors or benchtop bioreactors with working volumes on the order of 0.1 to 1 liter (often around 100 mL) are used in R&D and early process development. At this scale, researchers explore conditions and assess feasibility. There are even microbioreactors that hold only a few milliliters, used for high-throughput screening of cultures. These systems mimic larger bioreactors but on a miniature scale.
Bench scale (lab/pilot scale): Bioreactors in the liter range (e.g. 1 L, 5 L, 10 L, up to ~50 L) are used for more detailed studies and pilot production. A bench-scale bioreactor is often where scientists refine the process and gather data for scale-up. They might produce small batches of a product for testing. Academic and industrial labs often have bench-scale stirred-tank reactors that are modular and highly instrumented, allowing experimentation before going bigger.
Production scale (industrial scale): Large bioreactors have working volumes in the hundreds to thousands of liters. These are found in manufacturing facilities. For example, a production bioreactor for a commercial biopharmaceutical might be 1,000 L, 5,000 L, or even 15,000 L in volume. In vaccine manufacturing or commodity fermentation (like bioethanol), multiple 10,000+ L reactors may run in parallel.
These vessels are often stainless steel (for reuse and robustness). At production scale, maintaining uniform conditions throughout that huge volume is a challenge – engineers use scale-up principles to preserve mixing, oxygen transfer, and other conditions similar to the smaller scales. Often, intermediate pilot scale (e.g. a 100 L or 200 L bioreactor) is used as a stepping stone between bench and full production, to ensure the process behaves as expected when volumes increase.
No matter the scale, the fundamental principles are similar, just as a home brewing kit and a commercial brewery fermentation tank operate on the same concept. It’s important to note that scaling up is not simply a linear matter of making a tank bigger; factors like oxygen delivery, agitation, and heat removal become more complex with volume. Bioprocess engineers spend a lot of effort in scaling up bioreactors so that a cell culture that worked in a 2 L vessel will also perform in a 2,000 L vessel. Fortunately, the widespread use of standard stirred-tank designs means a well-characterized process can often be scaled with predictable results
Conclusion
Bioreactors are foundational technology in the biotech era, they enable us to grow cells on a useful scale, whether we want a small flask worth of cells for research or tens of thousands of liters for mass production. The choice of bioreactor type and setup depends on many factors (the organism, the product being made, process requirements, cost considerations, etc.), but all bioreactors serve the same basic purpose: to create an ideal, controllable home for cells to carry out biochemical reactions.
With advances in materials, sensor technology, and automation, bioreactors have become more efficient and specialized, driving forward innovations in medicine, food, energy, and environmental solutions. By understanding the basics of how bioreactors work and what they can do, we appreciate how vital these systems are, often quietly operating behind the scenes, in delivering many products and services that improve our lives. Bioreactors truly are the engines that power modern biotechnology, from the lab to the factory floor.
Frequently Asked Questions (FAQ)
It is a vessel that provides a controlled environment for growing cells and microorganisms.
The vessel, agitator, sparger, temperature control, sensors and aseptic ports.
Stirred-tank, airlift, photobioreactor and packed-bed designs.
Single-use systems use disposable bags without cleaning, while stainless steel systems are reusable and suit large volumes.
Microbes, mammalian and insect cells, plant cells, and algae.
References
Doran, P. M. (2013). Bioprocess Engineering Principles (2nd ed.). Academic Press.
Shuler, M. L., & Kargi, F. (2017). Bioprocess Engineering: Basic Concepts (3rd ed.). Pearson.
Stanbury, P. F., Whitaker, A., & Hall, S. J. (2016). Principles of Fermentation Technology (3rd ed.). Butterworth-Heinemann.
Harrison, R. G., Todd, P. B., Rudge, S. R., & Petrides, D. P. (2015). Bioseparations Science and Engineering (2nd ed.). Oxford University Press.
Eibl, R., Löffelholz, C., & Eibl, D. (2018). Single-Use Technologies in Biopharmaceutical Manufacture. John Wiley & Sons.
Enfors, S.-O., Jahic, M., Rozkov, A., Xu, B., Hecker, M., Jürgen, B., … Krull, R. (2001). Physiological responses to mixing in large-scale bioreactors. Journal of Biotechnology, 85(2), 175–185.
Posten, C., & Walter, C. (2012). Photobioreactors: Production systems for phototrophic microorganisms. Applied Microbiology and Biotechnology, 100(14), 6599–6614.
Barbosa, E. J., & Francisco, O. (2019). Advances in mammalian cell culture for vaccine production. Journal of Applied Microbiology, 127(2), 345–356.
U.S. Food and Drug Administration. (2015). Guidance for Industry: PAT – A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. FDA.









